
Shellfish Pathogens of Interest
Here is some information about the different pathogens (and diseases) the ADL focuses on! Some of the pathogens here we can routinely check for using some of our available diagnostic services, while others are currently being researched.

Perkinsus marinus
Dermo
Perkinsus marinus is a protozoan parasite that infects the eastern oyster (C. virginica) causing Dermo disease. This disease can lead to high mortality in both cultured and wild oyster populations. Infections are highest during the late summer through fall seasons (August-November) in New England waters.
Classification: Protozoan apicomplexan/dinoflagelate
Distribution: Gulf of Mexico to North Atlantic coasts
Transmission: Directly between oysters
Diagnosis: Ray's Fluid Thioglycollate, Histology, qPCR, PCR
Pathogenesis: The organisms are taken up from the water column through feeding, are phagocytized by host cells (hemocytes) and multiply inside them. After expansion and cell division, the organisms burst, damaging the host cell and leaving to find a new cell to repeat the cycle. The pathogen causes significant inflammation in the oyster leading to tissue/cell death and eventual parasitic anemia. As this happens, oyster tissues are replaced by growing numbers of the parasite and start to atrophy and become edematous. The disease is slow progressing and severe infection due to P. marinus is usually not apparent until several months later.
Haplosporidium nelsoni
Multinucleated Sphere Unknown (MSX)
Haplosporidium nelsoni, also known as MSX, is a spore forming protozoan parasite that infects the eastern oyster (C. Virginica). The organism targets gill tissue before rapidly spreading to cause a disseminated disease leading to inflammation, emaciation and eventually death in adult oysters. Mortality is highest during August/September but die-offs can occur from late summer through fall (August-November) in New England waters.
Classification: Spore forming protozoan
Distribution: Eastern coast of North America, Florida to Canada
Transmission: Indirect transmission. There is believed to be an intermediate host that houses the parasite before it becomes reproductively active, but none has been found at this time. Once the parasite is mature enough to reproduce, it is thought to leave the intermediate host and re-enter the water column where it infects the oyster.
Diagnosis: Histology, hemolymph screening, PCR, and qPCR
Pathogenesis: From the water column, MSX, in its infective stage, enters the host via water tube epithelium and initially infects the gill. The parasite replicates here in the gill tissues. When contained/localized to the gill, most oysters can survive just fine with MSX infection. The issues arises when there is systemic spread. Persistent infection and replication damages host cells and tissues. Eventually the parasite penetrates into the underlying tissues causing rapid spread of the infection throughout. The now systemic infection causes organ disruption and lysis of the tissues. Infected oysters can have feeding rates less than half of uninfected oysters causing emaciation. The disease can also severely impair gonadal development .

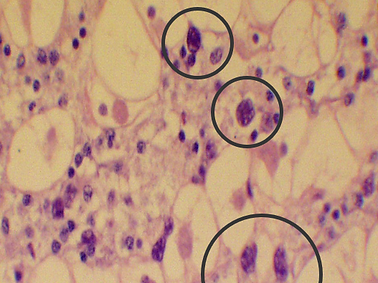
Haplosporidium costale
Seaside Organism (SSO)
Haplosporidium costale, also known as SSO, is a spore forming protozoan parasite that infects the eastern oyster (C. Virginica). SSO will be ingested from the water column and infect the host through tissues of the digestive tract where it will spread to connective tissues throughout, continuing with replication until sporulation. Mortality is highest in the spring and early summer (March-June) with synchronous sporulation occurring in May/June, usually killing the host due to parasite load. During fall and winter, low numbers of SSO are present with increasing infection in the spring followed by synchronous sporulation mentioned above. The organism is thought to need 2 hosts to complete its lifecycle, with one being the Eastern oyster and the other unknown.
Classification: Spore forming protozoan
Distribution: North Atlantic Coast, Virginia to Maryland
Transmission: Unknown
Diagnosis: Histology, PCR, and qPCR
Pathogenesis: From the water column, SSO is consumed via feeding and infects the digestive epithelium. From here it travels to the underlying connective tissues where it continues its lifecycle and replicates. The organism then lies dormant in low numbers waiting to sporulate in coming spring/summer. As the temperatures get warmer, the organism spreads, increasing the parasite load to a level as to disrupt tissue and organ function. When sporulation finally occurs in May/June, due to sheer number, it typically kills the host.
Roseovarius crassostreae
Roseovarius Oyster Disease (ROD), formerly
Juvenile Oyster Disease (JOD)
Roseovarius crassostreae is a bacteria that infects and causes mass mortality events in juvenile oysters (<1 yr). The organism deposits itself between shell and mantle layers and cause ulceration and necrosis of the shell. This leads to impaired growth, mantle retraction, unequal shell growth, inflammation and eventual mortality. Juveniles with shell heights measuring <25mm are the most heavily affected.
Classification: Alpha-proteobacteria
Distribution: Northeastern coastal areas (MA, RI, NY, and ME)
Transmission: Directly between oysters, water filtrate from contaminated oysters
Diagnosis: Gross exam, bacterial culture, PCR
Pathogenesis: The bacterial infection causes a progressive necrosis and ulceration of shell epithelium accompanied by severe inflammatory reactions. When located at the adductor muscle attachment, the muscle can detach from the shell, contributing to mortality factors. Other factors include myoepithelial degeneration and subsequent secondary infections. High mortality rates occur ~4-6 weeks after initial signs, but mortality can be seen as soon as 1 week after initial symptoms.


Quahog Parasite Unknown
(QPX)
Quahog parasite Unknown (QPX) is a protist belonging to the family Thraustochytriidae that causes morbidity and mortality in hard clams (Mercenaria mercenaria) along the northeast coast of North America. The disease most commonly targets 2-year-old cultured clams. The disease is recognized as focal swellings or nodules within the mantle tissue, found usually adjacent to the siphon. Within these nodules exists the pathogen which surrounds itself in a thick protective mucus layer to hide from a clam’s immune system. Sick clams will often be found at the surface instead of burrowed in the sand (although this is not pathognomonic for QPX, any pathogen causing stress and disease can cause clams to surface) and in densely populated areas of aquaculture.
Classification: Protist
Distribution: Northeastern Atlantic Coast, Virginia to Canada
Transmission: Directly between oysters
Diagnosis: Gross exam, bacterial culture, PCR
Pathogenesis: The bacterial infection causes a progressive necrosis and ulceration of shell epithelium accompanied by severe inflammatory reactions. Sometimes leading to adductor muscle detachment which contributes to mortality factors. Other factors include myoepithelial degeneration and subsequent secondary infections. High mortality rates occur ~4-6 weeks after initial signs, but mortality can be seen as soon as 1 week after initial symptoms.